Ensure early detection and proactive management of arrhythmias
Gaps in rehab sessions can lead to missed cardiac events. Frontier X Plus ensures continuous ECG tracking, enabling early detection, timely intervention, and reduced complications.
Distance from a facility is the biggest barrier to cardiac rehab, with only 24% of eligible patients completing it despite reimbursement being available. The Frontier X Plus, FDA 510(k) cleared ECG monitor bridges this gap by enabling virtual cardiac rehab with continuous ECG monitoring from home. Its wireless, adhesive-free design ensures all-day comfort, reducing the fatigue of traveling to a rehab center. By providing reliable, continuous insights, Frontier X Plus empowers both patients and healthcare providers. By reducing in-person clinic visits, it enhances compliance, improves recovery outcomes and access to quality rehab—anytime, anywhere.
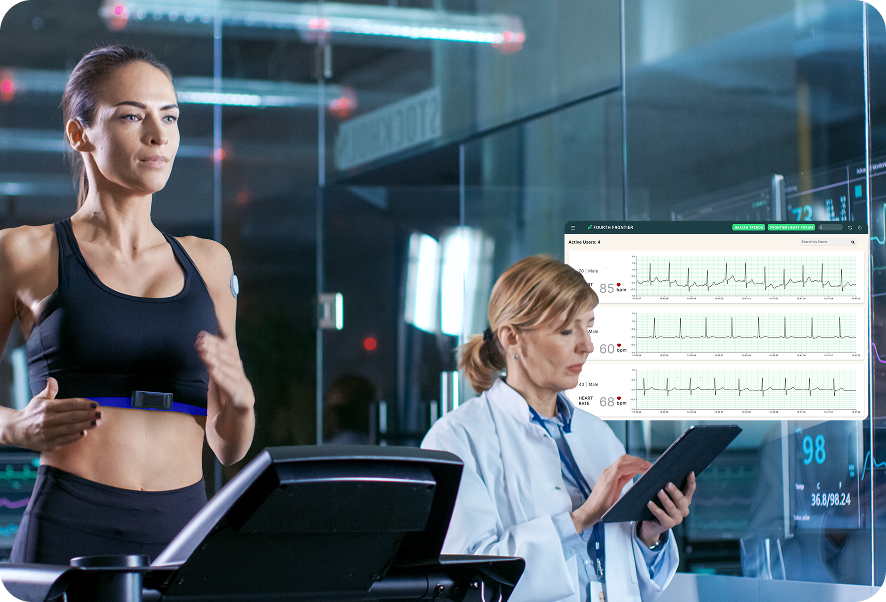
Distance from a facility is the biggest barrier to cardiac rehab, with only 24% of eligible patients completing it despite reimbursement being available. The Frontier X Plus, FDA 510(k) cleared ECG monitor bridges this gap by enabling virtual cardiac rehab with continuous ECG monitoring from home. Its wireless, adhesive-free design ensures all-day comfort, reducing the fatigue of traveling to a rehab center.

Gaps in rehab sessions can lead to missed cardiac events. Frontier X Plus ensures continuous ECG tracking, enabling early detection, timely intervention, and reduced complications.

Unlike traditional devices and patches, Frontier X Plus provides precise, reliable ECG signals with reduced motion artifacts, even during exercise.
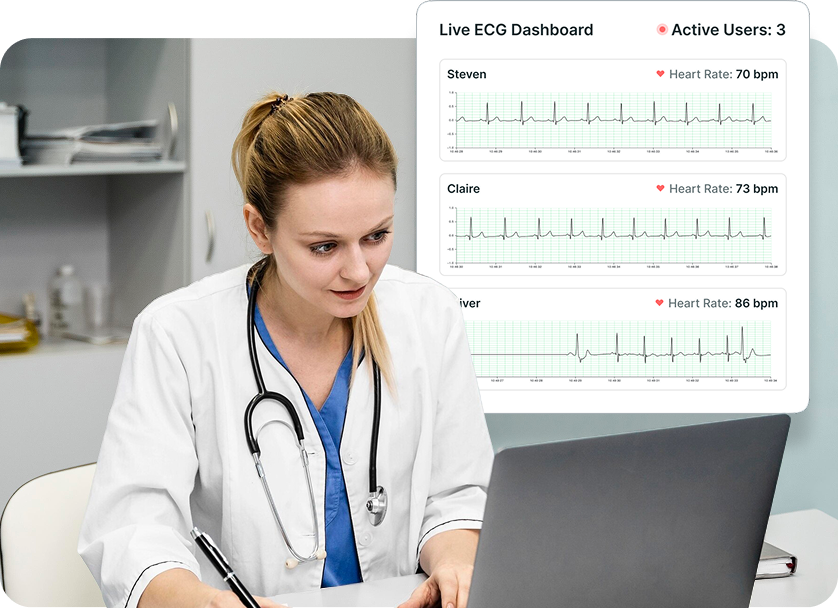
Frontier X Plus streamlines monitoring with a secure, centralized dashboard that provides continuous ECG data from multiple patients without requiring patients to visit the clinic.
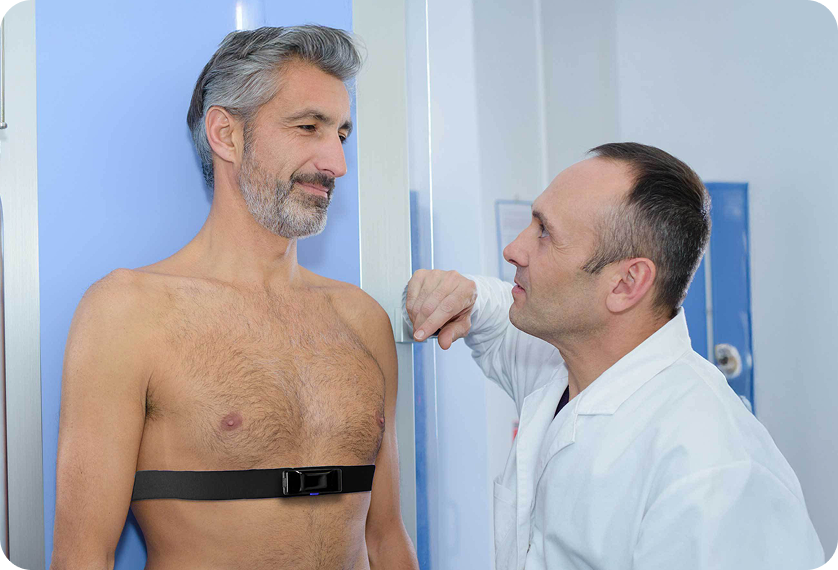
Unlike costly diagnostic equipment or single-use patches, it fits into existing infrastructure without capital investment, while expanding billing and reimbursement using CPT code 93798.









*Disclaimer: RoHS2 & Reach Compliant chest strap.


This study aimed to validate the Frontier X Plus (FX+) single-lead, continuous ECG device by comparing its performance against the traditional FDA Cleared 12-lead ECG in 832 patients in both a clinical and real world setting including individuals with and without atrial fibrillation (AFib). The performance of the Frontier X Plus in detecting atrial fibrillation (AF) demonstrated a sensitivity of 98.10% and a specificity of 97.88%, when compared to the paired gold standard device as adjudicated by 3 independent cardiologists.


A case report of a 16-year-old competitive runner experiencing sudden-onset tachycardia episodes, exceeding 200 bpm, documented using a Garmin Instinct™ watch. Despite normal cardiac evaluations, the patient later used a Frontier X™ ECG Fitness Tracker, which successfully captured heart rhythm data during episodes and helped monitor his response to beta blocker therapy.
The Frontier X device played a crucial role in continuously monitoring heart rate and respiratory rate, ensuring safety and enabling real-time feedback during the exercise sessions. The success of this remotely delivered intervention suggests that similar models could be effectively implemented for broader PH patient populations, offering a scalable alternative to hospital-based programs.
Note:
The Frontier X Plus (FX+) is a prescription only US FDA 510(k) cleared medical device for ambulatory ECG monitoring. It is not tested for use in individuals with pacemakers or ICDs. It is not meant for the diagnosis of cardiac ischemia. Please request the IFU for intended use, indications, contraindications and availability in your geography.
Disclaimer: All images used are for illustrative purposes only.
