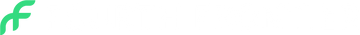
Frontier X+ is now
FDA 510(k) Cleared
For Cardiologists looking for a self-applied, out-of-the-box solutions for clinical practice. Our latest product, available on prescription, is a medical device that records and transfers single-channel ECG and provides automated detection of atrial fibrillation and other arrhythmias.
Are you a patient that’s interested? Register Here to learn how your healthcare team can give you a prescription.
Fourth Frontier is Trusted by Leading Cardiologists,
Cardiac Rehab Centers, and Researchers Globally
Fourth Frontier is Trusted by Leading Cardiologists, Cardiac Rehab Centers, and Researchers Globally
Applications across Cardiology, Rehabilitation, and Sports Medicine






All the Benefits of our Cutting-edge ECG Wearable
patient comfort and ease of use. No wires or adhesives, enhance patient comfort and ease of use.
monitoring in under 1 minute. Patients wear the device and begin monitoring in under 1 minute.
climate friendly solution.
atrial fibrillation and other arrhythmias.
Introducing the Frontier Platform
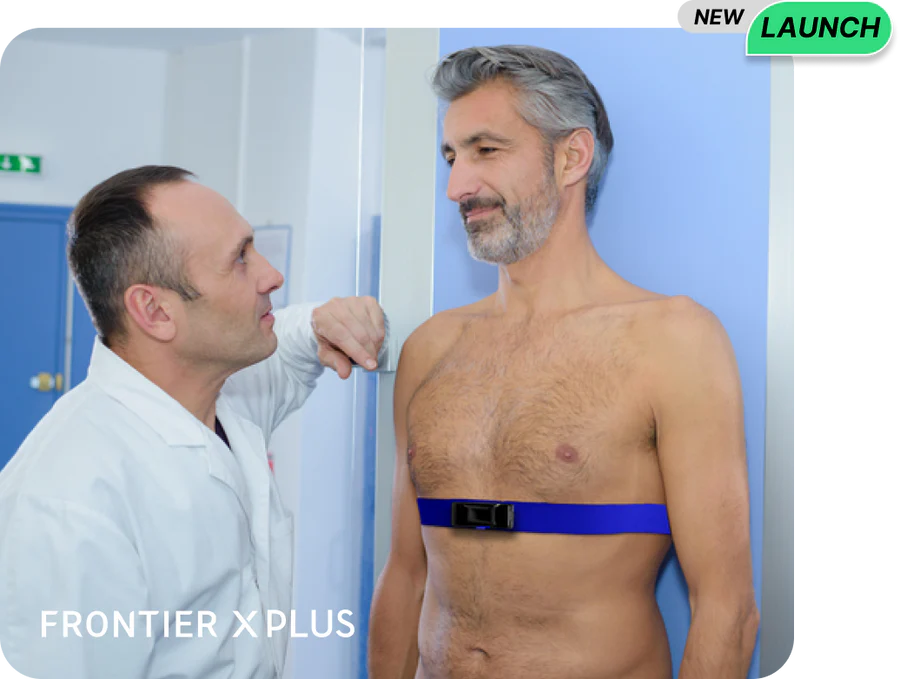
Multi-Patient Dashboard and ECG Stream
Monitor multiple users simultaneously with an easy-to-use dashboard. Live streaming of the ECG allows you to validate the quality of the signal remotely.

Hours. Months. Years of ECG Data - Streamlined
Use our tools for hospitals & researchers to manage multiple patient accounts. Receive notifications on activity and download raw ECG data.
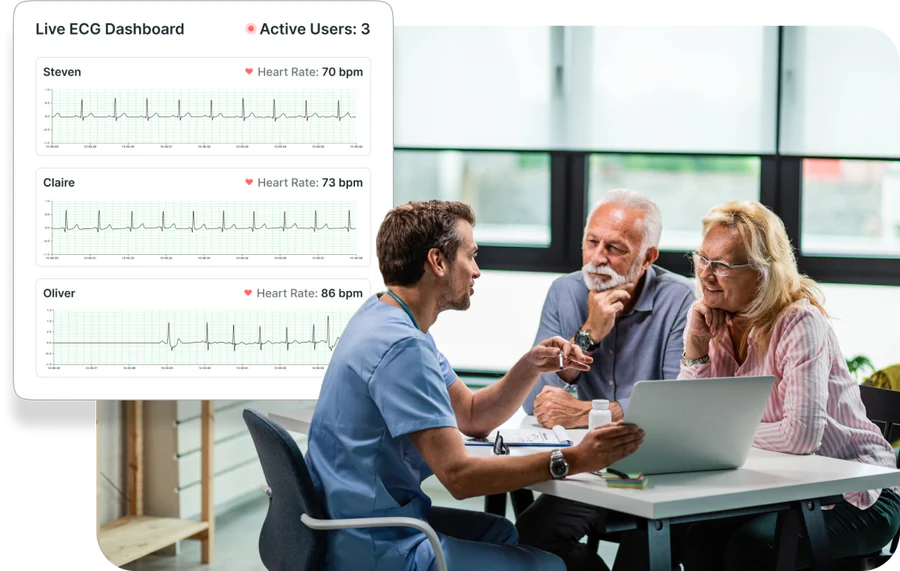
Visualise Trends, Predict Patient Needs
Summary trend lines of select metrics can support early decision making and refine decisions for your patients, employee groups, and trial participants.
Publications
Roberts, William O. MD, MS, FACSM1; Levine, Benjamin D. MD, FACSM2
A case report of a 16-year-old competitive runner experienced sudden-onset tachycardia episodes, exceeding 200 bpm, were documented using a Garmin Instinct™ watch. Despite normal cardiac evaluations, The patient later used a Frontier X™ ECG Fitness Tracker, which successfully captured heart rhythm data during episodes and helped monitor his response to beta blocker therapy.
Ciara McCormack, Brona Kehoe, Sarah Cullivan, Noel McCaffrey, Sean Gaine, Brian McCullagh, Andrew McCarren, Sarah J Hardcastle, Niall M Moyna
The Frontier X device played a crucial role in continuously monitoring heart rate and respiratory rate, ensuring safety and enabling real-time feedback during the exercise sessions. The success of this remotely delivered intervention suggests that similar models could be effectively implemented for broader PH patient populations, offering a scalable alternative to hospital-based programs.
Technical Safety and Performance Testing
Frontier X+ Device
- IEC 60601-2-47:2014 Medical Electrical Equipment Part 2-47: Particular Requirements for the safety. Including essential performance of ambulatory electrocardiographic systems.
- IEC 60601-1:2005, AMD1:2012 Medical Electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 +AMD1: 2020 CSV, Medical Electrical Equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances Requirements and tests.
- IEC 60601-1-11:2015 Medical Electrical Equipment - Part 1-11: General requirements for basic safety and essential performance - Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment.
Chest Strap
- Compliant with REACH and RoHS2 standards.
Clinical Validation
This study aimed to validate the Frontier X+ (FX+) single-lead, continuous ECG device by comparing its performance against the traditional FDA Cleared 12-lead ECG in 832 patients in both a clinical and real world setting including individuals with and without atrial fibrillation (AFib). The performance of the Frontier X+ in detecting atrial fibrillation (AF) demonstrated a sensitivity of 98.10% and a specificity of 97.88%, when compared to the paired gold standard device as adjudicated by 3 independent cardiologists.




Frontier Platform for Research
Unified cloud for ECG, participant data, research notes, and activity.
- Access to all your participant's account data registered under the admin account scalable to suit your study size and resources.
- Automatic notifications every time a user registers under the admin account and uploads their data.
- Stored and processed on HIPAA-compliant AWS servers.
Downloadable Raw Data
- Raw ECG data in CSV format with the raw voltage of the ECG signal sampled at 125Hz.
- CSV File for derived data in 20-second intervals - Heart Rate, Rhythm and HRV.
- ECG recordings available in EDF format.
- Selected segments of an ECG recording in a PDF format.
Customised support & service.
- Personal Account management and staff training.
- Free technical support.
Note:
The Frontier X Plus (FX+) is a prescription only US FDA 510(k) cleared medical device for ambulatory ECG monitoring. It is not tested for use in individuals with pacemakers or ICDs. It is not meant for the diagnosis of cardiac ischemia. Please request the IFU for intended use, indications, contraindications and availability in your geography.